BEYOND SUBSTANTIAL EQUIVALENCE : ETHICAL EQUIVALENCE
SYLVIE POUTEAU*
Journal of Agricultural and Environmental Ethics 13: 273-291, 2000
(Accepted June 6, 2000)
Kluwer Academic Publishers, http://www.wkap.nl/oasis.htm/274804
ABSTRACT : The concept of substantial equivalence, introduced for the risk assessment of genetically modified (GM) food, is a reducing concept because it ignores the context in which these products have been produced and brought to the consumer at the end of the food chain. Food quality cannot be restricted to mere substance and food acts on human beings not only at the level of nutrition but also through their relationship to environment and society. To make this context explicit, I will introduce an 'equivalence scale' for the evaluation of food chains (GM or not GM). By contrast with substantial equivalence, which involves mainly quantitative, analytical methods of evaluation, 'qualitative equivalence' refers to 'less' or non-substantial factors which require new methods of evaluation based on qualitative principles. 'Ethical equivalence' refers to factors that show the moral value contained in food products. To analyse the different levels at which ethics is needed in food chains, I will use the French principles: ‘Liberty, Equality, Fraternity’, or freedom, equality, solidarity, and add a fourth principle: sustainability. Sustainability, solidarity, and freedom can be applied to the evaluation of environmental, socio-economic, and socio-cultural ethical equivalence, respectively. Equality refers to justice and should operate so as to guarantee that sustainability, solidarity, and freedom are satisfied. I suggest that ethics can provide a basis for a renewal of the food chain concept. Besides Quality Assurance, it is now essential to develop an ‘Ethical Assurance’ and this equivalence scale could provide a basis to set up ‘Ethical Assurance Standards’ (EAS) for food chains.
KEY WORDS : Food Chain, Genetically Modified (GM) Food, Substantial Equivalence, ‘Qualitative Equivalence’, ‘Ethical Equivalence’, ‘Equivalence Scale’, Quality Assurance, ‘Ethical Assurance’
In a recent commentary entitled ‘Beyond substantial equivalence’ Millstone et al. (1999a) questioned the adequacy of the concept of substantial equivalence for the safety evaluation of genetically modified (GM) foods. Besides the insufficiency of this concept for risk assessment, there is currently no or little account of food quality criteria other than those that may constitute a direct threat on health. No concept exists so far to address food qualities that lay beyond substance and that may affect people in various ways. Thus, the question ‘Beyond substantial equivalence’ can also lead to another question: ‘Equivalence beyond substance’. Indeed, substantial equivalence, whether in its present form or in a more refined and scientific version, is calling for a non-substantial, social and ethical counterpart.
The concept of substantial equivalence was first described in 1993 and implemented in 1998 by the OECD (Organization for Economic Cooperation and Development). It was introduced to determine the safety of novel foods derived from modern biotechnology, i.e. mainly GM foods, by means of comparison with existing foods or food components with a known history of safe use (OECD, 1993). The comparison with natural non-GM counterparts was seen as a means to provide a guiding principle and useful tool for regulatory scientists engaged in safety assessments. It was endorsed in 1996 by the FAO (Food and Agriculture Organization) and the WHO (World Health Organization) who recommended that "safety assessment based upon the concept of substantial equivalence be applied in establishing the safety of foods and food components derived from genetically modified organisms" (FAO, 1996). Although substantial equivalence is not intended to substitute for a safety assessment, it has nevertheless been widely used as a reference for decision-making in official approval for the introduction of GM food. If a GM food can be characterized as substantially equivalent to is natural counterpart, it can then be assumed to pose no new health risks and hence to be acceptable for commercial use.
Millstone et al. argued that the concept of substantial equivalence is insufficient to ensure that novel GM foods are safe for human consumption. This is mainly due to the absence of a proper definition of the concept of substantial equivalence: ‘the degree of difference between a natural food and its GM alternative before its ‘substance’ ceases to be acceptably ‘equivalent’ is not defined anywhere, nor has an exact definition been agreed by legislators’. As a result, comparison tests carried out in GM and non-GM foods are usually limited to a restricted set of criteria, including chemical and nutritional evaluation with a set of selected substances and superficial animal testing. Because GM food’s toxicity cannot be predicted from its chemical composition, Millstone et al. (1999a) ‘believe that it [substantial equivalence] is misguided and should be abandoned in favor of one that includes biological, toxicological and immunological tests rather than merely chemical ones’.
Following the publication of Millstone et al.’s controversial commentary last October, many expressed their disagreement with its arguments in a number of letters and commentaries (Gasson, 1999; Miller, 1999; Tester et al., 1999; Trewavas et al., 1999). In turn, this led Millstone et al. to respond with an explanation of their position (Millstone et al., 1999b). Further informed scientific objections to substantial equivalence can also be found in Ho and Steinbrecher (1998). Their conclusions point to the context in which the concept of substantial equivalence was adopted by the FAO and the WHO. This includes preconceived claims in favor of genetic engineering, the obliteration of the distinction between genetic engineering and conventional breeding, and the failure to take existing scientific evidence pointing to hazards into account. They also insist on the lack of specification for the choice of a comparator and tests for establishing substantial equivalence, and on the restriction of scope which exempts known hazards from safety assessment, such as incidental residues resulting from the use of chemicals (pesticides or veterinary drugs) during food production or the possible emergence of food-borne pathogens. Also exempted are secondary, unintended effects so that safety assessment focuses almost exclusively on intended effects aimed at by the genetic modifications.
The aim of this paper is not to discuss further the inadequacy of substantial equivalence for risk assessment of GM food or its lack of scientific basis. Indeed, I would like to discuss substantial equivalence not from a risk assessment angle only but from a wider food quality perspective, including social and ethical issues. To this end, I will introduce ethical counterparts to the concept of substantial equivalence so as to provide another view of what can be seen ‘beyond substantial equivalence’. Here, the concept of equivalence can prove very useful. A main reason is that equivalence often seems to substitute for substantial equivalence, either literally for short (Husset, 1998) or implicitly as a thought content, so that other essential issues introduced by GM food are concealed and are not given sufficient attention. Indeed, if no difference is found between a GM food and its natural counterpart, they are then considered as ‘equivalent’. This applies especially to food components, such as soybean lecithin or refined oils, that can be refined to the point where it becomes difficult or impossible to trace their GM organism (GMO) origin through analytical methods. This has important consequences in terms of labeling since in such cases the relevance of GM food labeling has often been questioned. Another advantage of the concept of equivalence is that it requires the establishment of consensus references or standards as a basis for comparison. However, equivalence does not mean equality: it is clear that differences can and will be found between GM foods and their natural counterparts, whether at the level of substance or at the socio-economic and cultural levels. But a GM food cannot be rejected simply because differences exist. It is the impact of these differences that needs to be assessed.
The misuse of equivalence points to the fact that food quality cannot be restricted to mere substance and that food acts on human beings not only at the level of nutrition but also through their relationship to environment and society. Besides chemical, toxicological, and immunological issues, ethical issues should also be addressed. Beyond substantial equivalence, ‘qualitative equivalence’ and ‘ethical equivalence’ are to be found as ethical counterparts. I will also show that the concept of ethical equivalence is interesting because it can be applied to the whole of the food chain sector and can constitute a framework for the development of what I will call an ‘Ethical Assurance’.
SUBSTANTIAL EQUIVALENCE - THE 'NO-CONTEXT' VALUE
The concept of substantial equivalence derives straightforwardly from the thought processes of modern science. This is based on rationalizing reductionism in which objectification plays a prominent role (Ladrière, 1997). Subjective issues are obliterated or reduced to objective criteria. In his Discourse on method, published in 1637, Descartes (1987) pioneered this approach for living organisms and viewed them as sophisticated machines ruled by the laws of physics and chemistry. Since then, this approach has proved both convenient and productive. As a result, substance rather than process has become the main and often only focus of most scientific research. Along this line, the concept of substantial equivalence obviously concerns substance and relies only on objective criteria to estimate and measure risks by means of analytical methods. However, it also requires a probabilistic approach since it is usually clear that a nil risk cannot be guaranteed in risk assessment. If GM food had been treated in the same way as novel chemical compounds, such as pharmaceuticals, pesticides, and food additives, the acceptable daily intake would have been restricted to 1% of the human diet (Millstone et al., 1999a). Instead, the new regulation introduced by the European Commission in Regulation EC 49/2000 states that GM food labeling, in case of adventitious contamination, should be required only above a threshold of 1% of GM food components. GM food labeling and its control through GMO traceability are clearly associated with the concept of substantial equivalence. They constitute the ternary system by which the consumer is meant to be informed about GM food.
The concept of substantial equivalence, and its relatives – GM food labeling and GMO traceability –, is a blind concept because it does not require any specific knowledge other than food chemical composition. To put it into an extreme scenario, one could be given a flask containing a white substance in a black box. By using all sorts of sophisticated analytical methods, it could be deduced, for example, that the white substance is substantially equivalent to cows milk with such and such qualities, suggesting that it might have been produced in The Netherlands, etc... In other words, the concept of substantial equivalence deals with food products taken out of context, ignoring the way these products have been produced and brought to the consumer at the end of the food chain. The concept of substantial equivalence is the context-less value of food products. It draws a veil between the consumer and the process that led to the food products. Through this concept, the distance introduced between the consumer and the producer by the complexity of modern food chains is magnified to the point where the process and context behind the food products are obliterated. Of course, this obliteration of context does not apply only to the concept of substantial equivalence. But this concept, and the way GM food is dealt with generally, can prove useful in drawing attention to more general dead ends in our modern food chains and thus can act as a magnifying lens to reveal them.
FOOD CHAIN PROCESS - THE MEANING OF CONTEXT
Although substantial equivalence can prove useful in providing some information for the risk assessment of new foods, it also brings confusion and leads to endless discussions as to what the number and refinement of laboratory analyses should be (OECD, 1998). Whatever the sophistication of the concept of substantial equivalence might be, this would have to confront the fact that objective criteria are unable to encompass or substitute for subjective – i.e. based on individual perception – criteria. Ultimately, safety is a relative notion and risk assessments should at some point come to terms with the perception and acceptability of risk by people. Both are essentially dependent on the context in which risks may be experienced by individuals. For this reason, Käppeli and Auberson (1997; 1998) suggest that an evaluation based on tolerability criteria would be more appropriate than the probabilistic approach of risk assessment for determining the safety of transgenic plants.
Food products also mean processes. The content of food is more that its chemical composition and in the case of GM food, the public debate reaches far beyond food risk assessment. Behind substance, many other aspects are involved and can constitute or be perceived as risks or hazards for the consumer. The food chain process is the context. And the context can provide answers to questions such as who did what and how, when, and where, etc...? Context, the often forgotten factor when dealing with living processes, from genetics (Holdrege, 1996) to human activities, needs to be re-introduced by considering these other aspects that are necessary to get from substantial equivalence to equivalence per se.
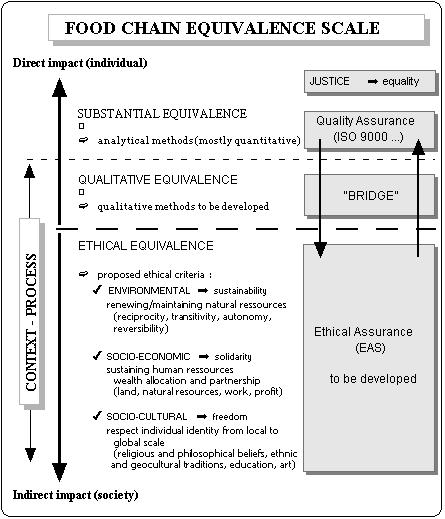
Substantial equivalence is not sufficient to account for any risk or hazard that can be introduced for human beings through biotechnology, whether directly or indirectly. Directly refers to risks that may be encountered when GM food is actually consumed. Indirectly refers to risks generated whether or not the GM food product is actually taken in and consumed. Direct risks can more readily be established or perceived because there is a direct connection between the individual and the food product that may cause harm. Indirect risks correspond to those risks that may affect society at large, for example pollution or unemployment, and thus would affect the individual as a consequence. These distinctions are helpful when designing an 'equivalence scale' for the GM and the non-GM food chains (see diagram). Besides substantial equivalence, other equivalence concepts need to be introduced.
Even within the limited range of direct risks, the concept of substantial equivalence appears insufficient to account for context within food products. By contrast with substantial equivalence, which mainly involves quantitative analytical methods of evaluation, I will use the

Diagram.
term 'qualitative equivalence' to refer to non-substantial or 'less' substantial factors. These would include for example, the country of origin of the food product, the agricultural methods of production (use of chemical fertilizers, pesticides, herbicides for crops, of antibiotics and hormones for animals, etc...), the mode of harvest (in season and maturity harvest or post-harvest maturation and storage), the methods of conservation (radiation, pasteurization, heat treatment, etc...), the processing of food (heat treatment, methods of extraction, purification, etc...), etc... Some of these factors can be approached through analytical methods but most of them would escape evaluation through existing methods. New, global methods based on qualitative principles need to be developed to measure the non-substantial quality of products. One known qualitative method with a long history of use for wine evaluation is the palate of oenologists. Perhaps this will help people to realize that quality is a human notion and that no existing analytical machine can be as sophisticated as human faculties!
Qualitative equivalence can help to re-introduce the historical dimension of food products. But as yet, its social implication is limited. To fully lift the veil, it is necessary to go one step further and consider the many indirect risks that may be brought to human beings by food products. I will use the term 'ethical equivalence' to refer to these indirect factors because these will show the moral value contained in food products.
ETHICAL EQUIVALENCE: SUSTAINABILITY, SOLIDARITY, FREEDOM, AND EQUALITY
Ethics encompasses principles that are generally accepted as fair and desirable for society. This requires that consensus including all individuals be established in an informed purposeful way and not taken for granted. The French philosopher Ricoeur (1990) offers a definition of ethics as the 'aiming toward a fair life with and for others within fair institutions'. This may also be expressed by the French republican motto 'Liberty, Equality, Fraternity'. I will use these principles to identify the different levels at which ethics is needed in food chains and to establish a scale for ethical equivalence. For this purpose, I will add a fourth principle, sustainability, which is necessary for the maintenance and perpetuation of the first three principles with time in order to confer on them a transgenerational dimension. For each principle, I will also examine how GM foods fail to meet a number of ethical criteria.
Partly as a response to the negative perception of GM technology by the public, there has been recently a growing concern of the professional sector (academics, industries, and legislators) about issues related to the ethical use of biotechnology. So far, apart from implicit ethical references in discussions, few explicit and decision-making initiatives can be reported. The first international conference of the Council of Europe on ‘Ethical issues arising from the application of biotechnology’ was held last year and is expected to promote the development of a European ethical convention (Kinderlerer, 2000). The European Association for BioIndustries (EuropaBio), totaling more than 600 small and medium sized companies involved in biotechnology, has recently approved and published a set of core ethical values (Fears and Tambuyzer, 1999). However, ethical issues often seem to be addressed through the various questions and fields of application of biotechnology rather than by the use of comprehensive leading principles. I believe that concepts and thought contents should be worked out thoroughly and be made explicit in order that ethics can become effective and not merely the compilation of good intentions, actually often constrained by so-called ‘compelling pragmatic realities’. I will thus attempt to investigate how the principles of sustainability, solidarity (fraternity), freedom (liberty), and equality can operate to shed light on ethical issues associated with GM food and beyond, to the whole of the food chain sector.
In contrast to liberty, equality, and fraternity, which relate to partnership between members of society, sustainability introduces a notion of partnership between society and nature or environment and living organisms (micro-organisms, plants, and animals). Sustainability requires the establishment of a natural contract which is based on: reciprocity, for example, domesticated animals, crops and human beings are mutually dependent on each other for their subsistence; transitivity or distributiveness, i.e. benefits at one level are not detrimental for other levels; autonomy through diversity, i.e. the whole of the food chain is flexible enough to support a wide range of changes; and reversibility, i.e. detrimental effects, if any, are reversible. The notion of sustainability can be used for the evaluation of environmental ethical equivalence in the food chains by assessing the way natural resources are maintained and renewed and living organisms are preserved. This applies to soil and water resources, natural ecosystems, biodiversity, landscape, animal welfare, etc... In other words, the environmental impact of foods chains should be evaluated at all levels of the process, so as to draw up an environmental balance sheet associated with food
Sustainability and GM food
Claims in favor of GM crops often involve environmental benefits for agriculture through reducing the need for agri-chemicals and promoting the practice of sustainable agriculture. However, these statements are based on a reductive view of sustainability since a global environmental assessment is usually missing. Current engineered pest resistance relies on vulnerable, monogenic mechanisms that would rapidly result in the selection of resistant pests and would need to be replaced in the short term. Herbicide resistance is prone to similar drawbacks. Emergence of weeds resistant to herbicide through outbreeding and gene dispersal would impose the replacement of both the transgenic crops carrying the herbicide resistance gene and the herbicide itself. Instead of sustainability, these approaches promote transient, disposable pest management systems with short-lived, ephemeral crop varieties and herbicides or pesticides.
Digenic systems are expected to delay the emergence of pest resistance. But the question is: for how much longer? In addition, every pest type (insects, fungi, bacteria, nematodes...) would have to be addressed through specific digenic systems, raising the number of genes to be introduced. To herbicide and pest resistance genes should then be added genes for more efficient nitrate assimilation or for nitrogen fixation and reduced nitrate accumulation in crops; genes for a better control of water exchanges and/or drought resistance, to name but a few. Every single environmental nuisance is addressed independently from the others and no comprehensive vision is applied to foresee the impacts at different levels. Indeed, the possibly detrimental consequences of combining several foreign genes in crop genomes are usually overlooked. Moreover, with a larger number of genes to be introduced, it is likely that the number of varieties generated will drop, leading to reduced genetic diversity. In seeking for the one ideal system to solve any problem, the reshaping of the genetic make-up of crops is intrinsically aiming at uniformity. Indeed, GM crops currently grown over vast areas in the United States are highly uniform.
Finally, a major difficulty with the environmental claims in favor of GM crops is that they are based on the assumption that efficient agriculture is necessarily detrimental to the environment and that biotechnology, combined with sustainable farming, is our best hope for the future (Trewavas, 1999). However, this holds true only for intensive agriculture, which results in a decline in carbon and nitrogen content of soil, in nitrogen leaching out into rivers and natural water, in natural water wastage, in increased susceptibility to devastating diseases and in intensive resort to pesticide treatments to compensate for lack of genetic diversity and adequate agricultural operations (Matson et al., 1997; Tilman, 1998). To think in terms of eliminating pesticides from food is merely a reduction. Indeed, disease cannot be considered in isolation from the rest of the farming system. In organic farming for example, most crop protection actually happens as a by-product of operations carried out for other reasons, including planting and sowing techniques, crop rotation, etc... Not least is the use of landraces that provide genetic diversity and reduced susceptibility to crop pests (for a comparison of pest management between sustainable agriculture and biotechnology, see Brown, 1998). It should be added that improvement of soil fertility and reduced detrimental effects on the environment are also important issues on the agenda of organic farming (Drinkwater et al., 1998; Tilman, 1998). Piling up new technologies to counter adverse effects of the old ones is one option. But to attack problem at the root is another option that seems to some extent more promising and predictable in terms of sustainability. This requires that more effort and funding be allocated to the research and development of alternatives such as organic agriculture rather than to technology that aims to compensate for nuisance generated by intensive agriculture. It is interesting to note that the first seed breeding program that aims to produce varieties for organic farming has been recently announced (Coghlan, 2000).
Sustainability can also be applied to the socio-economic domain, in terms of human resources. This applies to employment, salary, income, insurance, retailing, local economic activities, etc... But solidarity (fraternity) appears more essential as a ruling principle for socio-economic activities since the basic question concerns wealth allocation (land, natural resources, work, and profit).
The purpose of economic activities is to provide the goods and services that are necessary for society, whether they are needed by all or by minorities as in the case of special diets. The collective interest is to produce goods of the best quality in sufficient quantity for those who need them. Work is the means by which this purpose is achieved through economic deeds. Profit is the outcome of the social contract between members of the society; it arises as a consequence of the fact that an individual does not have to produce all that he needs on his own but contributes to fulfil the needs of others through socio-economic partnership. The way profit is distributed is thus of the outmost importance and should be the highest expression of this partnership. Only the lack of a global view or a lack of morality at one stage or another can explain misery within society. Unemployment constitutes a denial of this social contract because it deprives unemployed individuals of the rights to contribute to economic solidarity and endorse their full social responsibility. Competition for the sake of economic solidarity rather than for the sake of profit should stimulate evolution and generate economic vitality and creativity. It is the responsibility of every actor and stakeholder of the food chain that through their work and deeds economic solidarity is achieved, by the sharing of benefits and work, etc... In other words, the global socio-economic impact of food chains should not be evaluated merely in terms of national gross profit, for example, but rather in terms of economic solidarity or shared profit between the different actors from producers to consumers.
Solidarity and GM food
Claims in favor of GM crops often invoke their contribution to economic growth and potential benefits for developing countries. The development of agri-biotechnology has created new jobs in research and development areas, start-up companies, etc... However, this development cannot be considered outside the general context of economic evolution, from the local to the global scale, and from the possible indirect influences of this development on other activities or on other parts of the world, such as developing countries. For example, the engineering of natural dyes, essential oils, additives, or drugs, by cloning of the corresponding genes may deprive developing countries of resources based on the trade and export of raw or more or less processed traditional products. This can be interpreted both as resource and cultural piracy (i.e. taking the biological and natural resources and heritage of communities without permission) and economic piracy (destruction of domestic markets). There is a wide consensus that piracy should be stopped (Kinderlerer, 2000). However, this needs to be guaranteed in a more explicit way. In addition, in many of these countries seed is free and available at no cost. It is thus difficult to see how poor farmers would afford the costs of GM seed.
The patenting of the potential applications of natural species and genes is very similar in practice to the patenting of these natural species or genes themselves because the uses that can be made of them are usually self-contained in the properties of the species or the function of the genes. Indeed, they are usually drawn straightforwardly from them (medicinal species, genes encoding resistance or enzymes, etc…). Patenting is the foundation core of GM agri-business which thus intrinsically aims to impose a block to the free access to natural resources. Using this approach, the biotechnology company Monsanto in the United States put clauses in farmers' contracts constraining them to renounce the right to re-use the seed harvested. ‘Terminator technology', currently not deployed by Monsanto in response to a widespread public concern, constitutes a technological culmination of this trend: in place of heavy and expensive surveillance of contracting farmers, it relies on the introduction of a genetic system which has no other function than to be deleterious to the seed harvested.
Finally, the claim that GM crops are required to solve the problem of hunger in the world (Mann, 1999; Nuffield Council on Bioethics, 1999; Trewavas, 1999; Flavell, 2000) bypasses the fact that the main cause of hunger is not lack of food but politics (Christian Aid, 1999; Verheye, 2000). There is in fact enough food to feed all human beings in the world. It is doubtful that a doubling of food supplies would be much help in decreasing the high proportion of malnourished people world-wide. In fact, new markets are actually sought for agricultural surplus, such as biomass and fuel, plastics, industrial oils, or medical and veterinary applications (Dunwell, 1999; Zechendorf, 1999). In itself, this clearly demonstrates that food quantity is not a major cause of concern. Agricultural surplus and hunger together can justify every possible application of GM technology. But genuine, effective measures against hunger will have to await ethical, political decisions.
Freedom (or liberty) is an essential principle of the socio-cultural life. This includes religious and philosophical beliefs or opinions, local traditions, ethnic and geocultural habits, education, science, and art, ... The notion of freedom can be applied to the evaluation of socio-cultural ethical equivalence by assessing the way the identity and cultural choices of individuals are respected from the local to the global scale. Again, this implies that any level of the food chains should comply with the criteria so as to allow the determination of a comprehensive ethical bottom line.
Freedom and GM food
Against the principle of freedom, GMOs are often presented as an inevitable if not the only way ahead in agriculture to solve starvation, pollution by agri-chemicals, etc... However, this fundamentalism in leading to uniformity is potentially a threat to diversity, sustainability, and solidarity (see above). Moreover, fundamentalism constitutes a threat for society and democracy and could endanger the socio-cultural life if individual choices are not respected. For example, implicit fundamentalism justifies the absence of GM food labeling. This was simply thought unnecessary in the United States until recently (Haslberger, 2000). In Europe, although the principle of labeling was accepted a few years ago, its implementation is still way behind (Michael, 1999). Although the vast majority of the European population is reluctant to accept GM food, many food products that contain GM ingredients are currently imposed on them with little possibility of choice and information. Because technical, objective scientific arguments are meant to be the only valid arguments, public suspicion of GMOs is interpreted as irrational and based on ignorance. But studies by the Economic and Social Research Council (ESRC, 1999) show that the public is not stupid and ignorant in its approach to risks, and that it has a qualified understanding of the main problems.
Labeling means that segregation can be made between food that is derived from GMOs and food that is not. However, the European Commission Regulation EC49/2000 does not impose labeling if no DNA or protein is present in food products and if less than 1 % of GM ingredients has been adventitiously introduced. In addition, the use of GM technology at any stage of food production, such as in animal feed, does not have to be labeled under the current regulation. For these reasons, although the regulation of GM food labeling has been improved in recent Regulations such as EC49/2000, it is in fact promoting the setting up of an expensive default labeling for food that does not contain GM ingredients. Indeed, if the proof has to be made that contamination below a threshold of 1 % was not deliberate, it would probably be more profitable for actors of the food chain to engage further in the process and to certify the absence of GM ingredient in food products. Obviously, deliberate addition of GM ingredients can be easily demonstrated and traced whilst the absence of GM ingredients is more difficult to prove due to the costs or to lack of appropriate methods of detection. This means that the costs of traceability and labeling will probably have to be supported by those who want to stay with traditional products. As a consequence, traditional products will be more expensive and not everybody can afford them anymore!
In analyzing the needs of developing countries, possible technological improvements are rarely combined with socio-economic and cultural assessment of the acceptability and impact on the populations concerned. Because in Southeast Asia 70 % of children under the age of five suffer from vitamin A deficiency, engineered rice with improved provitamin A is expected to save many lives and improve staple food nutrition for half of the world’s population who eat rice daily (Guerinot, 2000; Ye et al., 2000). But how this golden rice would be accepted by the population as regard to its color, GM nature, and other possible alterations, has simply not been considered, so the basic philosophy often seems to be : ‘people will take it if we tell them it is good for them’. Nor has this technology been compared to other alternatives that would be better adapted to local traditions and, moreover, would involve nutrition and farming education of the population. Rice is not the cause of vitamin A deficiency. But the way it is complemented with other food products may constitute causes of any imbalance. Simple alternatives are probably not investigated because the successful introduction of three genes encoding the required enzymes for provitamin A synthesis in rice is a technical tour de force (Ye et al., 2000), so golden rice simply has to be the only option...
Now where does the equality principle operate ? The domain where all individuals are equal is the law domain, or justice. Justice or equality should operate so as to guarantee that sustainability, solidarity, and freedom, i.e. the three degrees of ethical equivalence : environmental, socio-economical, and socio-cultural, are satisfied. Ethics does not need to be imposed through imperatives and prescriptions or to become compulsory under the law, nor would this be desirable or akin to its essence. In addition, the field of application of ethical principles in food chains is extremely vast and involves many diverse actors across all scales, from local to global. Because the roles and capacities of governments in enforcing ethical, environmental, or social regulations have decreased due to growing globalization and transnational corporations, it is unlikely that ethical practice in food chains will be achieved without active involvement of the private sector.
Already a number of companies and corporations have set up their own company code of ethical practice and there has been recently a growing concern for ethical issues amongst private corporations. The incorporation of these issues is viewed as a means (i) to self-regulate their activities rather than be subjected to legislative constraints, (ii) to anticipate rapid changes in technological development and society’s response to them, and (iii) to investigate new profitable ways of reducing socio-economic and environmental impacts within the existing economic system (Fears and Tambuyzer, 1999; Daily and Walker, 2000). This has led to a number of initiatives and co-operative engagement within the corporate sector, addressing mainly environmental issues but also integrating a wide array of social and economic considerations, such as health, safety, and social equity (Daily and Walker, 2000).
A number of frameworks and standards have emerged in the past few years to make ethical values operational in the corporate sector. These include the Eco-Management Audit Scheme (EMAS) in Europe and the International Standards Organization (ISO) 14001 in the United States which comprise environmental standards, and the Social Accountability (SA) 8000 which encompass social standards relative to work conditions in different countries (Daily and Walker, 2000). As mentioned above, EuropaBio has recently agreed and published a set of core ethical values for biotechnological industries which include general principles as well as health, agriculture, food, and environment considerations (Fears and Tambuyzer, 1999). It is the responsibility and choice of any company and corporation to subscribe to these standards and to qualify for certification. The role of institutions is to guarantee that regulations and certifications are respected, especially when the distance between the different actors does not allow direct assessment by individuals. And it is the role of justice to ensure that any claim of ethical practice by a company is liable to prosecution in case of corruption or deviation from the subscribed standards, so ethics does not become merely a fashionable and marketable product.
TOWARD AN ‘ETHICAL ASSURANCE’ IN FOOD CHAINS
Starting from substantial equivalence and GM food, the implication of ethical equivalence leads us to wider questions in connection with some of the difficulties currently encountered in our modern food chains. Here, I suggest that ethics can provide a basis for a renewal of the food chain concept. Through global transparency, food chain ethics will allow consumers to become 'consum-actors', in other words to take on individual and collective responsibility when confronted with choices the implications of which reach far beyond the mere expression of a need or satisfaction. In the past few years, much effort has been applied in developing Quality Assurance in industry. Among the numerous standards evolved by ISO, the ISO 9000 created in 1987 is most widely used. However, the term ‘Quality Assurance’ is misleading since it is clear that the public is expecting more from food quality than mere safety and nutritional criteria. Paradoxically, Quality Assurance owes more to analytical and quantitative evaluation than to qualitative evaluation as such. In addition, the perception of quality by people is continuously evolving. The surge of quality issues has created a new conceptual field of reflection which is now calling more extensive research and clarification. To account for an enlarged range of quality criteria, a number of ethical standards have started to emerge recently (see above). This is also influencing Quality Assurance as shown by the move from product to process-based standards in the current revisions of ISO 9000, which may be seen as an important step in taking the context into consideration. The purpose of these revisions is to make the ISO 9000 compatible with the more recent, environmental ISO 14000. But it might soon become difficult to claim quality without taking into account environmental issues and, thus, to keep apart quality standards and environmental standards. In addition, other ethical issues are awaiting further consideration. For these reasons, I believe that it is now time to let ‘Ethical Assurance’ find its place in our food industry. Ethical assurance standards (EAS) would complement Quality Assurance and include a set of ethical values, some of which may be found in existing ethical standards.
To introduce Ethical Assurance in food chains, an ethical norm needs to be set up with a hierarchy of criteria. Equivalence can prove a useful concept to establish a scale for these criteria, starting from substantial equivalence and qualitative equivalence with no or little ethical implication to environmental, socio-economic, and socio-cultural ethical equivalence (see diagram). Substantial equivalence, in calling for analytical methods of evaluation, is clearly relevant to Quality Assurance. Qualitative equivalence needs to be addressed through qualitative methods but these methods are dramatically missing at the moment. Their development should contribute to the acknowledgement of ethical issues as well as the linking together technical and ethical issues, often a cause of disagreement and misunderstanding in the public debate. It is likely that this could also help to clarify the GM food debate – as an indirect benefit of substantial equivalence. Finally, EAS applicable to environmental, socio-economic, and socio-cultural ethical equivalence could be set up based on the principles presented in this paper: sustainability, solidarity, freedom, and equality. Across the whole scale, at every equivalence level, justice should guarantee that regulations are applied and that certifications are valid. It is worth noting that an ethical matrix based on a number of principles (well-being, autonomy, and justice) has also been proposed recently by the Food Ethics Council in the United Kingdom (1999).
The introduction of Ethical Assurance in food chains is of paramount importance and no doubt it will take time before a consensus is found about what ethical criteria should be and how to define EAS. It is a task of economic and social ethics (Arnsperger, in press) as a discipline and field of research to help provide a framework for this purpose. Considering the complexity of this task, transdisciplinary co-operation is a prerequisite in the scientific approach and economists, sociologists, lawyers, biologists, chemists, ecologists, etc... should be involved. But most importantly, food chain actors are expected to engage in the process, so that a partnership with farmer associations, food industry and retailers, consumer associations, and shareholders is established. Through transdisciplinary co-operation and engagement of the corporate sector, ethical assurance in food chains can be expected to progress on a sound basis.
ACKNOWLEDGEMENTS
This work was done as a contribution to the ETHOS group on Economic and Social Ethics of INRA, http://www.inra.fr/Internet/Directions/SED/EES/
I am grateful to Dominique Vermersch and Emmanuel Jolivet (ETHOS group, INRA) for their support and helpful discussions. I would also like to acknowledge Anne-Lucie Wack (ETHOS group, CIRAD) for fruitful collaboration on food chain ethics. Finally, I would like to thank the Ifgene (International Forum for Genetic Engineering) group for their help and support, and I am specially thankful to David J. Heaf (Ifgene UK coordinator) for his comments and corrections of the manuscript.
To F.S.C.
REFERENCES
Arnsperger, C., and P. Van Parijs (2000), Éthique Économique et Sociale. Paris: La Découverte, série Repères.
Brown, J.K.M. (1998), "How to Feed the World, in Two Contradictory Lessons", Trends in Plant Sciences 3, pp. 409-410.
Christian Aid (1999), Selling Suicide Farming, False Promises and Genetic Engineering in Developing Countries, Report, London.
Coghlan, A. (2000), "Seeds of Change", The New Scientist 29 January, p. 12.
Daily, G.C., and B.H. Walker (2000), "Seeking the Great Transition", Nature 403, pp. 243-245.
Descartes, R. (1987), Discourse on Method and The Meditations, London: Penguin Classics.
Drinkwater, L.E., P. Wagoner, and M. Sarrantonio (1998), "Legume-Based Cropping Systems Have Reduced Carbon and Nitrogen Losses", Nature 396, pp. 262-264.
Dunwell, J.M. (1999), "Transgenic Crops: The Next Generation, or an Example of 2020 Vision", Annals of Botany 84, pp. 269-277.
ESRC (1999), "The Politics of GM Food : Risk, Science and Public Trust", Environment Change Programme, www.gecko.ac.uk.
FAO (1996), Biotechnology and Food Safety, Report, Rome, http://www.fao.org/
Fears, R. and E. Tambuyzer (1999), "Core Ethical Values for European Bioindustries", Nature Biotechnology 17, pp. 114-115.
Flavell, R.B. (2000), "Plant Biotechnology. Moral Dilemmas", Current Opinion in Plant Biology 3, pp. 143-146.
Food Ethic Council (1999), Novel Food: Beyond Nuffield, Report, www.users.globalnet.co.uk/~foodeth
Gasson, M.J. (1999), "Genetically Modified Foods Face Rigorous Safety Evaluation", Nature 402, p. 229.
Guerinot, M.L. (2000), "The Green Revolution Strikes Gold", Science 287, pp. 241 and 243.
Halsberger, A.G. (2000), "Monitoring and Labeling for Genetically Modified Products", Science 287, pp. 431-432.
Ho, M.-W. and R.A., Steinbrecher (1998) "Fatal Flaws in Food Safety Assessment: Critique of the Joint FAO/WHO Biotechnology and Food Safety Report", Physicians and Scientists for Responsible Application of Science and Technology (PSRAST) homepage, http://www.psrast.org/
Holdrege, C. (1996), Genetics and the Manipulation of Life: The Forgotten Factor of Context, Hudson: Lindisfarne Press.
Husset, M.-J. (1998), L'Opinion Publique Face aux Plantes Transgéniques, Paris: Albin Michel, pp. 110-117.
Käppeli, O. and L. Auberson (1997), "The Science and Intricacy of Environmental Safety Evaluations", Trends in Biotechnology 15, pp. 342-349.
Käppeli, O. and L. Auberson (1998), How Safe is Safe Enough in Plant Genetic Engineering ?", Trends in Plant Sciences 3, pp. 276-281.
Kinderlerer, J. (2000), "Is a European Convention on the Ethical Use of Modern Biotechnology Needed ?", Trends in Biotechnology 18, pp. 87-90.
Ladrière, J. (1997), L'Éthique dans lUunivers de la rationalité, Namur: Artel-Fides.
Mann, C.C. (1999), "Crop Scientists Seek a New Revolution", Science 283, pp. 310-314.
Matson, P.A., W.J. Parton, A.G. Power, and M.J. Swift (1997), "Agricultural Intensification and Ecosystem
Properties", Science 277, pp. 504-509.
Michael, A. (1999), "'GM-Free' Food Labels are Value-Free", Nature Biotechnology 17, p. 420.
Miller, H.I. (1999), "Substantial Equivalence : Its Uses and Abuses", Nature Biotechnology 17, pp. 1042-1043.
Millstone, E., E. Brunner, and S. Mayer (1999a), "Beyond 'Substantial Equivalence'", Nature 401, pp. 525-526.
Millstone, E., E. Brunner, and S. Mayer (1999b), "Seeking Clarity in the Debate over the Safety of GM Foods", Nature 402, p. 575.
Nuffield Council on Bioethics (1999), Genetically Modified Crops: The Ethical and Social Issues, Report, http://www.nuffield.org.uk/bioethics/
OECD (1993), Safety Evaluation of Foods Derived by Modern Biotechnology - Concepts and Principles. Report, Paris.
OECD (1998), OECD Workshop on the Toxicological and Nutritional Testing of Novel Foods, Report, Aussois.
Ricoeur, P. (1990), Soi-Même Comme un Autre. Paris: Seuil.
Tester, M., S.L. Taylor, and S.L. Hefle; Ho, M.W. (1999), "Seeking Clarity in the Debate over the Safety of GM Foods", Nature 402, p. 575.
Tilman, D. (1998), "The Greening of the Green Revolution", Nature 396, pp. 211-212.
Trewavas, A. (1999), "Much Food, Many Problems", Nature 402, pp. 231-232.
Trewavas, A. and C.J. Leavert, "Conventional Crops are the Test of GM Prejudice"; Kearns, P. and P. Mayers, "Substantial Equivalence is a Useful Tool"; Burke, D. "No GM Conspiracy" (1999), Nature 401, pp. 640-641.
Verheye, W.H. (2000), "Local Farmers would be Able to Feed Africa if They were Given the Chance", Nature 404, p. 431.
Ye, X., S. Al-Babili, A. Klöti, J. Zhang, P. Lucca, P. Beyer, and I. Potrykus (2000), "Engineering the Provitamin A (Beta-Carotene) Biosynthetic Pathway into (Carotenoid-Free) Rice Endosperm", Science 287, pp. 303-305.
Zechendorf, B. (1999), "Sustainable Development : How can Biotechnology Contribute ?", Trends in Biotechnology 17, pp. 219-225.
Ifgene wishes to thank the author of this article for giving permission to republish it on this site.
The URL of this page is pouteaueng.htm